Drugs Commonly Used in Urology
August 25, 2019
Medications used to treat skin conditions include topical and oral drugs
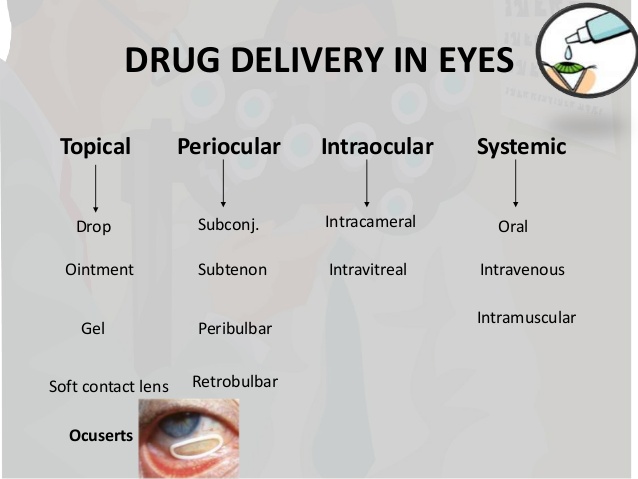
August 26, 2019FDA Approved Drugs for Ophthalmology
Drugs Approved in 2019
Rocklatan (netarsudil and latanoprost ophthalmic solution); Aerie Pharmaceuticals; For the treatment of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension, Approved March 2019
Drugs Approved in 2018
Oxervate (cenegermin-bkbj) ; Dompe Pharmaceuticals; For the treatment of neurotrophic keratitis, Approved August 2018
Drugs Approved in 2017
Luxturna (voretigene neparvovec); Spark Therapeutics; For the treatment of vision loss due to confirmed biallelic RPE65-mediated inherited retinal disease, Approved December 2017
Rhopressa (netarsudil ophthalmic solution) ; Aerie Pharmaceuticals; For the treatment of glaucoma or ocular hypertension, Approved December 2017
Vyzulta (latanoprostene bunod ophthalmic solution); Bausch & Lomb; For the reduction of intraocular pressure in patients with open-angle glaucoma or ocular hypertension, Approved November 2017
Zerviate (cetirizine ophthalmic solution 0.24%); NicOx; For the treatment of ocular itching associated with allergic conjunctivitis, Approved May 2017
Drugs Approved in 2016
Humira (adalimumab); Abbvie; For the treatment of uveitis, Approved July 2016
Xiidra (lifitegrast); Shire; For the treatment of dry eye disease, Approved July 2016
Drugs Approved in 2015
Eylea (aflibercept); Regeneron Pharmaceuticals; For the treatment of diabetic retinopathy of all stages, Approved March 2015
Drugs Approved in 2014
Eylea (aflibercept); Regeneron Pharmaceuticals; For the treatment of diabetic macular edema, Approved July 2014
Hetlioz (tasimelteon); Vanda Pharmaceuticals; For the treatment of non-24-hour sleep-wake disorder in the totally blind, January 2014
Omidria (phenylephrine and ketorolac injection); Omeros; For use during eye surgery to prevent intraoperative miosis and reduce post-operative pain, Approved June 2014
Oralair (Sweet Vernal, Orchard, Perennial Rye, Timothy and Kentucky Blue Grass Mixed Pollens Allergen Extract); Greer Labs; For the treatment of grass pollen-induced allergic rhinitis with or without conjunctivitis, Approved April 2014
Drugs Approved in 2012
Cystaran (cysteamine hydrochloride); Sigma Tau Pharmaceuticals; For the treatment of corneal cystine crystal accumulation due to cystinosis, Approved October 2012
Eylea (aflibercept); Regeneron Pharmaceuticals; For the treatment of macular edema following retinal vein occlusion, Approved September 2012
Jetrea (ocriplasmin); Thrombogenics; For the treatment of symptomatic vitreomacular adhesion, Approved October 2012
Lucentis (ranibizumab injection); Genentech; For the treatment of diabetic macular edema, Approved August 2012
Zioptan (tafluprost ophthalmic solution); Merck; For the treatment of elevated intraocular pressure, Approved February 2012
Drugs Approved in 2011
Eylea (aflibercept); Regeneron Pharmaceuticals; For the treatment of neovascular (wet) age-related macular degeneration, Approved November 2011
Drugs Approved in 2010
Xeomin (incobotulinumtoxinA); Merz Pharmaceutical; For the treatment of cervical dystonia and blepharospasm, Approved July 2010
Zymaxid (gatifloxacin ophthalmic solution); Allergan; For the treatment of bacterial conjunctivitis, Approved May 2010
Drugs Approved in 2009
Acuvail (ketorolac tromethamine); Allergan; For the treatment of pain and inflammation following cataract surgery., Approved July 2009
Bepreve (bepotastine besilate ophthalmic solution); Ista Pharmaceuticals; For the treatment of itching associated with allergic conjunctivitis, Approved September 2009
Besivance (besifloxacin ophthalmic suspension); Bausch & Lomb; For the treatment of bacterial conjunctivitis, Approved June 2009
Ozurdex (dexamethasone); Allergan; For the treatment of macular edema following branch retinal vein occlusion or central retinal vein occlusion, Approved June 2009
Zirgan (ganciclovir ophthalmic gel); Sirion Therapeutics; For the treatment of acute herpetic keratitis, Approved September 2009
Drugs Approved in 2008
Akten (lidocaine hydrochloride); Akorn; For anesthesia during ophthalmologic procedures, Approved October 2008
Astepro (azelastine hydrochloride nasal spray); Meda Pharmaceuticals Inc; For the treatment of seasonal and perennial allergic rhinitis, Approved October 2008
Durezol (difluprednate); Sirion Therapeutics; For the treatment of inflammation and pain associated with ocular surgery, Approved June 2008
Drugs Approved in 2007
AzaSite (azithromycin); InSite Vision; For the treatment of bacterial conjunctivitis, Approved April 2007
Drugs Approved in 2006
Lucentis (ranibizumab); Genentech; For the treatment of neovascular (wet) age related macular degeneration, Approved June 2006
Drugs Approved in 2004
Macugen (pegaptanib); Pfizer / Eyetech Pharmaceuticals; For the treatment of wet age-related macular degeneration., Approved December 2004
Drugs Approved in 2002
Restasis (cyclosporine ophthalmic emulsion); Allergan; For the treatment of low tear production., December 2002
Drugs Approved in 2001
Lumigan (bimatoprost ophthalmic solution); Allergan; For the reduction of intraocular pressure in patients with open-angle glaucoma or ocular hypertension, Approved March 2001
Travatan (travoprost ophthalmic solution); Alcon; For the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension, Approved March 2001
Valcyte (valganciclovir HCl); Roche; For the treatment of cytomegalovirus retinitis in patients with AIDS, Approved March 2001
Drugs Approved in 2000
Betaxon; Alcon; For lowering IOP in patients with chronic open-angle glaucoma or ocular hypertension, Approved February 2000
Quixin (levofloxacin); Santen; For treatment of bacterial conjunctivitis, Approved August 2000
Rescula (unoprostone isopropyl ophthalmic solution) 0.15%; Ciba Vision; For the treatment of open-angle glaucoma or ocular hypertension, Approved August 2000
Visudyne (verteporfin for injection); QLT; For the treatment of wet age-related macular degeneration (wet AMD), Approved April 2000
These drugs are distributed in Richmondhill by:
Dr. Berhooz Azizi
Drugs Approved in 1999
Alamast; Santen; pemirolast potassium ophthalmic solution, Approved September 1999
ZADITOR; Ciba Vision; Treatment for the prevention of itching of the eye, Approved July 1999
Drugs Approved in 1998
Alrex; Bausch & Lomb, Pharmos; Treatment for seasonal allergic conjunctivitis, Approved March 1998
Cosopt; Merck; Treatment for glaucoma or ocular hypertension, Approved April 1998
Lotemax; Bausch & Lomb, Pharmos; Treatment for post-operative eye inflammation, Approved March 1998
Salagen Tablets; MGI Pharma; Treatment for Sjogren’s Syndrome, Approved February 1998
Viroptic; King Pharmaceuticals; Treatment for inflammation of the cornea in children due to herpes simplex virus, Approved February 1998
Vitravene Injection; Isis Pharmaceuticals; Treatment for CMV in AIDS patients, Approved August 1998
Drugs Approved in 1997
Acular (ketorolac tromethamine ophthalmic solution) 0.5%; Allergan; Treatment for postoperative inflammation in patients who have undergone cataract extraction, Approved January 1997
Acular (ketorolac tromethamine ophthalmic solution) 0.5%; Allergan; Treatment for post-surgical inflammation following cataract extraction, Approved November 1997
BSS Sterile Irrigating Solution; Alcon; Treatment during ocular surgical procedures, Approved December 1997
Drugs Approved in 1996
AK-Con-A (naphazoline ophthalmic); Akorn; Over-the-counter combination vasoconstrictor/antihistamine product for opthalmic use, Approved January 1996
Alphagan (brimonidine); Allergan; Treatment for open-angle glaucoma and ocular hypertension, Approved September 1996
Ocuflox (ofloxacin opthalmic solution) 0.3%; Allergan; Treatment for corneal ulcers, Approved May 1996
OcuHist; Pfizer; Over-the-counter antihistamine eye drop, Approved January 1996
Vistide (cidofovir); Gilead; Treatment for cytomegalovirus (CMV) retinitis, Approved June 1996
Vitrasert Implant; Chiron; Drug delivery system for the treatment of cytomegalovirus, Approved March 199